- Hydrophilic character of carboxylic acid group and hydrophobic character of hydrocarbon chain.
- Low water solubility as the hydrocarbon chain character is greater than the carboxylic carbon character.
- Solubility decreases when there is a longer hydrocarbon chain.
- Lipids with a single carbon-carbon bonds (saturated) have a minimum melting temperature around 30 and increases with the length of the chain.
- Saturated fatty acids are commonly present in solid state at room temperature.
- Melting point of lipids that have one or more double carbon-carbon bonds (monounsaturated and polyunsaturated) have a lower melting point.
- Monounsaturated and polyunsaturated fatty acids are commonly present in liquid state at room temperature.
- Unsaturated fatty acids have weaker London Dispersion forces.
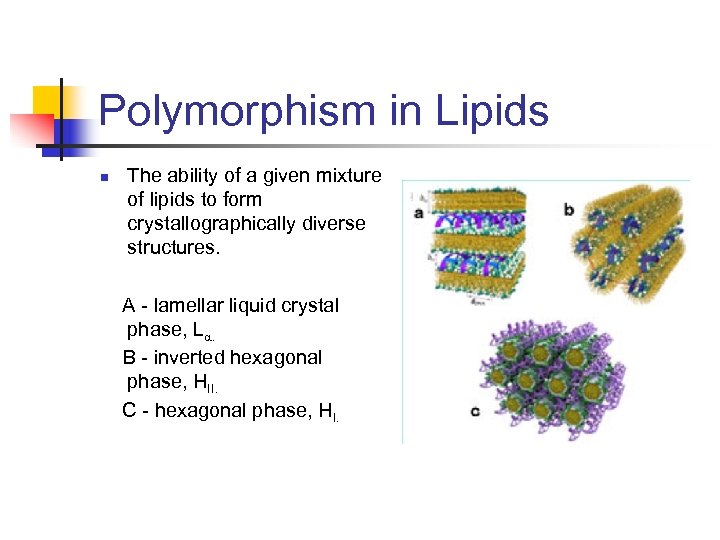
- Lipids are polymorphs, they have different forms when they packed themselves or form crystalline structures.
Bibliography:
Comentarios
Publicar un comentario